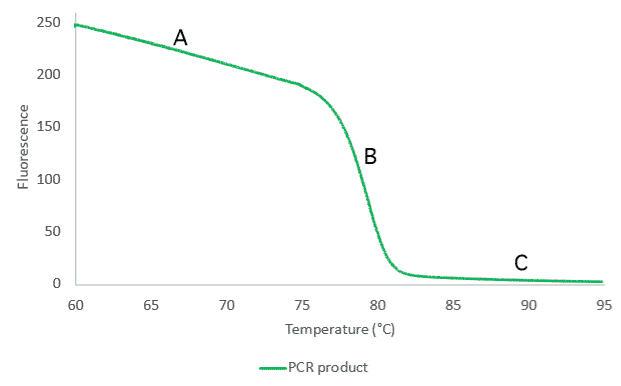
The most commonly used method to determine the melting temperature of a PCR product is to subject the product to a temperature gradient in the presence of intercalating dye.The dyes are chemicals that only emit light when bound to double stranded DNA. In a typical melting experiment, a PCR product is mixed with an intercalating dye, and the fluorescence emitted by this mix is monitored as the sample is slowly heated.The outcome of the analysis is a curve displaying fluorescence changes emitted by the sample over the range of temperature that the sample was subjected to, commonly referred to as a melting profile (Figure 1).
At the beginning of the melting experiment the temperature is low and all PCR product in the sample is double stranded. Thus, we observe high levels of florescence from the sample (Figure 1 – A). We continue to observe high levels of fluorescence, as the temperature increases up to the point, where all hydrogen bonds within the PCR fragment are broken and the amount of double stranded PCR product drastically decreases. Consequently, we observe a sharp decrease in the detected fluorescence level (Figure 1 – B).
At a high temperature there is no double stranded PCR product in the sample and the fluorescence levels are close to 0 (Figure 1 – C). The temperature at which we observe the sharp drop in the fluorescence depends on the number of hydrogen bands in the analyzed PCR product and hence is specific to analyzed fragment.This type of analysis can concurrently be performed on standard RT- qPCR instrument as a majority of those instruments have built in modules that allow post-PCR melting analyses.
Figure 1:
Learn more about MethylDetect DNA Methylation Kits here.
