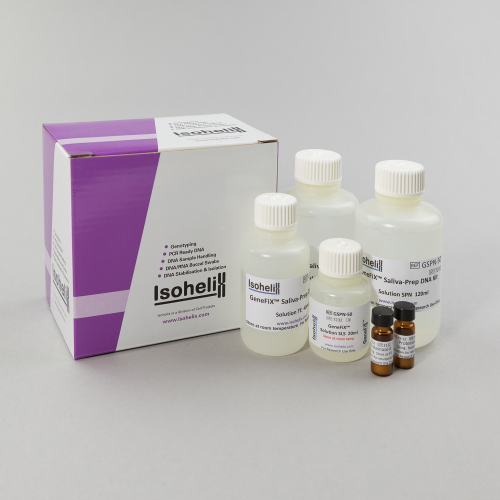
GeneFix Saliva-Prep 2 DNA Isolation Kit
The GeneFiX Saliva-Prep 2 Isolation kit is specifically designed for use with saliva samples. It uses a new formulated precipitation method to quickly and easily isolate DNA from samples up to 2ml in the GeneFiX Saliva DNA Collectors.
This kit does not use solvents, columns or filtration. Separate protocols are provided for use with GFX-01, GFX-02 and GFXA-01 saliva collectors. The reduced number of steps makes it ideal when a simple and efficient isolation method is required.
- High yields of over 100µg from 2 ml of saliva
- Fully optimized for use with saliva
- Fast handling times and reduced handling steps
- No solvents, columns or filtration requirements
- DNA isolation from saliva
- GSPN-02 - 2 reactions with 2ml samples
- GSPN-12 - 12 reactions with 2ml samples
- GSPN-50 - 50 reactions with 2ml samples
Kit Contents:
- Proteinase K
- Solution SPN
- Solution TE
- Solution SLS
If the yield and/or purity are not as expected, please check these steps for the best results:
Insufficient Concentration
- Follow the second precipitation step in the protocol and rehydrate in a smaller volume of buffer.
Low Apparent Yield
- Recheck the PK and incubation time. Should be minimum 10µl PK/1ml of sample, and minimum 30 minutes. 60 minutes is optimum.
- After adding TE buffer, was the sample well vortexed, and allowed to stand for a minimum of 5 minutes? It is important to allow the DNA time to rehydrate before moving to the next step.
- If the sample is too concentrated, it can give the appearance of low yield. Try adding more TE (there should be at least 100µl per 1ml original sample), vortex and allow 5 minutes to rehydrate.
Low Purity
- Follow point 1 above
- Recheck that the RCF/g and spin times were correct. After the SPN and spin step, check that ALL liquid is removed, as this contains impurities. There is an additional brief spin and liquid removal step, be sure that all liquid is discarded.
- After spinning the sample in TE buffer at the second centrifugation step, ensure that none of the pellet is carried over to the clean tube, as the pellet contains impurities.
- For an accurate reading, use 2µl of sample for the nanodrop analysis.
Agarose Gel shows streaks
- Check loading volume – ideally use 0.1-0.2µg/DNA/mm of gel lane width. More than this overloads the gel and gives a streaked appearance.
- During the DNA purification, you must allow minimum 5 minutes in TE buffer for the DNA to rehydrate.
- If the sample is too concentrated, it can give the appearance of low yield and also streaky gels due to supercoiled DNA. Try adding more TE, vortex and allow 5 minutes to rehydrate.
- Check the collection date and expiry date of the sample. The sample should have been collected before the expiry date on the tube, and the collection date should be within 5 years. If the sample has been frozen, check whether it has been thawed and re-frozen, as this can cause DNA shearing.
Boca Scientific is your premiere source for high-quality, innovative solutions for Cell Biology, Molecular Biology, Immunology, genetics and other lab products and reagents. We bring leading-edge products from our own-line and around the world to laboratories in the US and Canada. Our goal is to offer excellent solutions to drive research and discoveries backed by superior customer support.

 GeneFix 1ml DNA/RNA Saliva Collectors
GeneFix 1ml DNA/RNA Saliva Collectors GeneFix 1ml Assisted DNA/RNA Saliva Collectors
GeneFix 1ml Assisted DNA/RNA Saliva Collectors