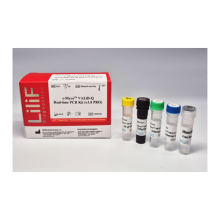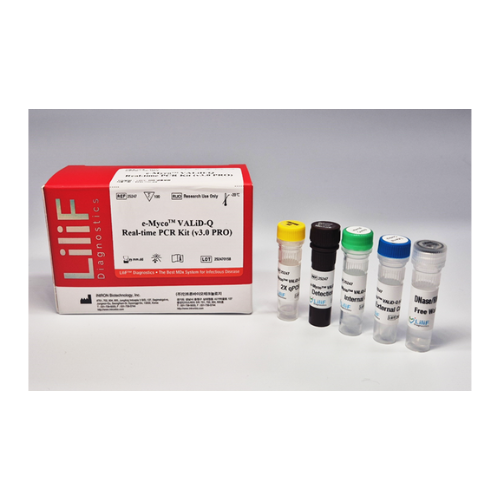
e-Myco™ VALiD-Q Real-time PCR Kit (v3.0 PRO)
The e-Myco™ VALiD-Q Real-time PCR Kit (v3.0 PRO) provides a speedy, reliable, and guideline-compliant method to detect mycoplasma contamination in cell-based manufacturing processes. The assay offers comprehensive coverage of international pharmacopeia standards (EP, USP, JP, and Korea MFDS), enabling simultaneous detection of compendial reference species within a single integrated panel. Panelized primers and probes, developed through extensive sequence alignment and in-silico validation, ensure high specificity and meet established detection limits without cross-reactivity with non-target microorganisms.
▶ Full compliance with Major 4 pharmacopeias : High performance and compliance
▶ Just 53 minutes : Far beyond the limitations of culture methods
▶ Internal Control (IC) and External Control (EC) ensure process validity across all steps, from nucleic acid extraction to PCR amplification

B, (Insect, Plant, or Avian-derived species) : In the Japanese Pharmacopoeia (JP), species of insect or plant origin (e.g., Spiroplasma citri) should also be tested in addition to the listed species. When avian cells or materials are used, avian-derived species (e.g., Mycoplasma synoviae) may also be considered for inclusion in the test panel.

Storage: - 20°C
- Fast, Strong, Sensitive, and fully validated
- Rapid PCR Detection: 53 minute real-time PCR detection of Mycoplasma and related Mollicutes class
- Targets a highly conserved 16S rRNA gene region for sensitive qualitative detection
- Validation for the production of Biopharmaceuticals & Advanced Therapy Medicinal Products(ATMP)
- Application of IC & EC system to verify the validity of qPCR
- Mycoplasma detection in cell cultures
- 2X qPCR mix 500 μl × 2 tubes
- Detection Solution 280 μl × 1 tube
- Internal Control 150 μl × 2 tubes
- External Control 25 μl × 3 tubes
- DNase RNase free water 1,000 μl × 1 tube
- Quick guide
Boca Scientific is your premiere source for high-quality, innovative solutions for Cell Biology, Molecular Biology, Immunology, genetics and other lab products and reagents. We bring leading-edge products from our own-line and around the world to laboratories in the US and Canada. Our goal is to offer excellent solutions to drive research and discoveries backed by superior customer support.

 e-Myco PLUS Mycoplasma PCR Detection Kit
e-Myco PLUS Mycoplasma PCR Detection Kit e-Myco VALID Mycoplasma PCR Detection Kit
e-Myco VALID Mycoplasma PCR Detection Kit